Nada Salem, Chemistry editor
The race to develop vaccines for COVID-19 marks the beginning of a new chapter in medicine: one where we have solutions for previously incurable diseases, more precise therapies, and phenomenally faster drug development. New frontiers in ribonucleic acid (RNA) medicine play a prominent role in this development.
Ribonucleic acid is a short stretch of genetic code. Different types of RNA perform different functions within cells and can be used in RNA medicine to encode new proteins, silence harmful genes, or attach to proteins to influence their function. Although RNA medicine has been the focus of intense research over the last few decades, it has only just begun to achieve its commercial potential.
A quick search of science journals reveals countless papers on RNA medicine that propose innovative solutions to long-standing problems. Some of these revolutionary medicines are already making their way through clinical trials. They include immunotherapy treatments for HIV, gene therapies for curing sickle cell disease, and personalized cancer vaccines. In 2018, the U.S. Food and Drug Administration approved the first RNA medicine for use in the United States.
Recently, one class of RNA medicine has attracted a lot of media attention: mRNA vaccines. An mRNA vaccine gives our bodies a code that tells cells how to make a particular protein – and relies on the cells to do the rest. Messenger RNA (mRNA) is an essential intermediary for translating DNA into functional proteins. Since our cells constantly generate proteins from mRNA, they already have the infrastructure to translate the code provided by the vaccine into proteins. By harnessing our cells as mini-factories, mRNA vaccines bypass the need for complex manufacturing in the lab and the risks associated with using whole viruses. These next-generation vaccines significantly reduce the time, money, and resources needed for vaccine development.
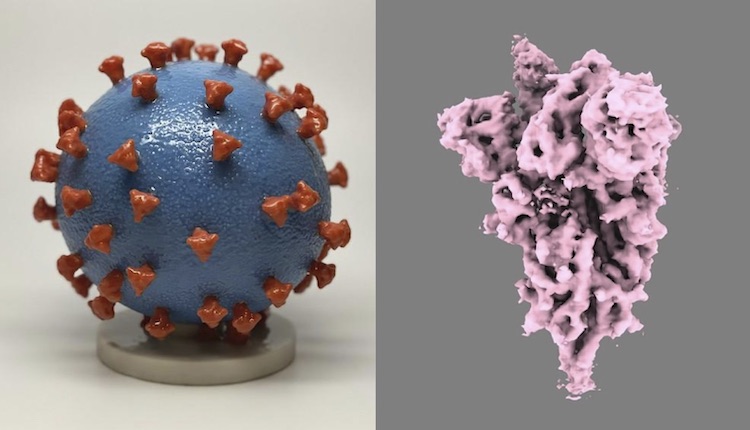
Instead of using whole viruses (left), new COVID-19 vaccines use mRNA that encodes the spike protein on SARS-CoV-2 (right). Spike proteins generated by our cells using mRNA are enough to develop immune recognition of the full virus. Images from U.S. National Institutes of Health.
Messenger-RNA technology also allows scientists to begin working on vaccines without handling a virus in the lab. Indeed, when Wuhan scientists released the full genome sequence of the COVID-19 virus on January 11, 2020, vaccine development started brewing halfway across the world just two days later – well before the virus was declared a global health emergency. Scientists will now be able to develop vaccines for future pandemicswithout knowing which viruses will cause them.
However, mRNA vaccines could not have become a reality without a simple yet powerful tool. There are two main challenges facing makers of mRNA vaccines. First, upon entering the body, mRNA must be delivered to its destination. This is challenging because it only lasts a few minutes in circulation before being digested by enzymes. Second, free mRNA is not taken up by target cells in sufficient quantities to have the desired effect.
To put mRNA in stealth mode, scientists engineered lipid nanoparticles. A lipid nanoparticle is a nano-sized bubble (a thousand times smaller than a dust particle) that acts as a Magic School Bus, carrying mRNA to where it needs to go. Lipid nanoparticles serve the dual function of shielding mRNA from degradation and facilitating its entry directly into cells. Lipid nanoparticles are made of lipids, organic compounds such as fats and oils that are insoluble in water. Since our cell membranes consist mainly of lipids, these molecules are excellent candidates for sneaking mRNA past the body’s defences unnoticed.
The pH-sensitive lipid nanoparticles used in COVID-19 vaccines are composed of several lipids, each with a crucial function in the vaccines’ stability and potency. The pH sensitivity of specific lipids allows the nanoparticle to release its safely packaged mRNA at precisely the right time and in the right place. Lipids on the nanoparticle’s surface become positively charged when they come into contact with an endosome – an acidic pocket that transports material within cells. The interaction with the acidic endosome disrupts the lipid nanoparticle membrane and releases the packaged mRNA into the cell. Imagine an Amazon package delivered straight through your front door, unwrapped in your kitchen, and ready to be used without you having to do anything. In the COVID-19 vaccines, the freed mRNA translates into spike proteins that help generate immunity against COVID-19.
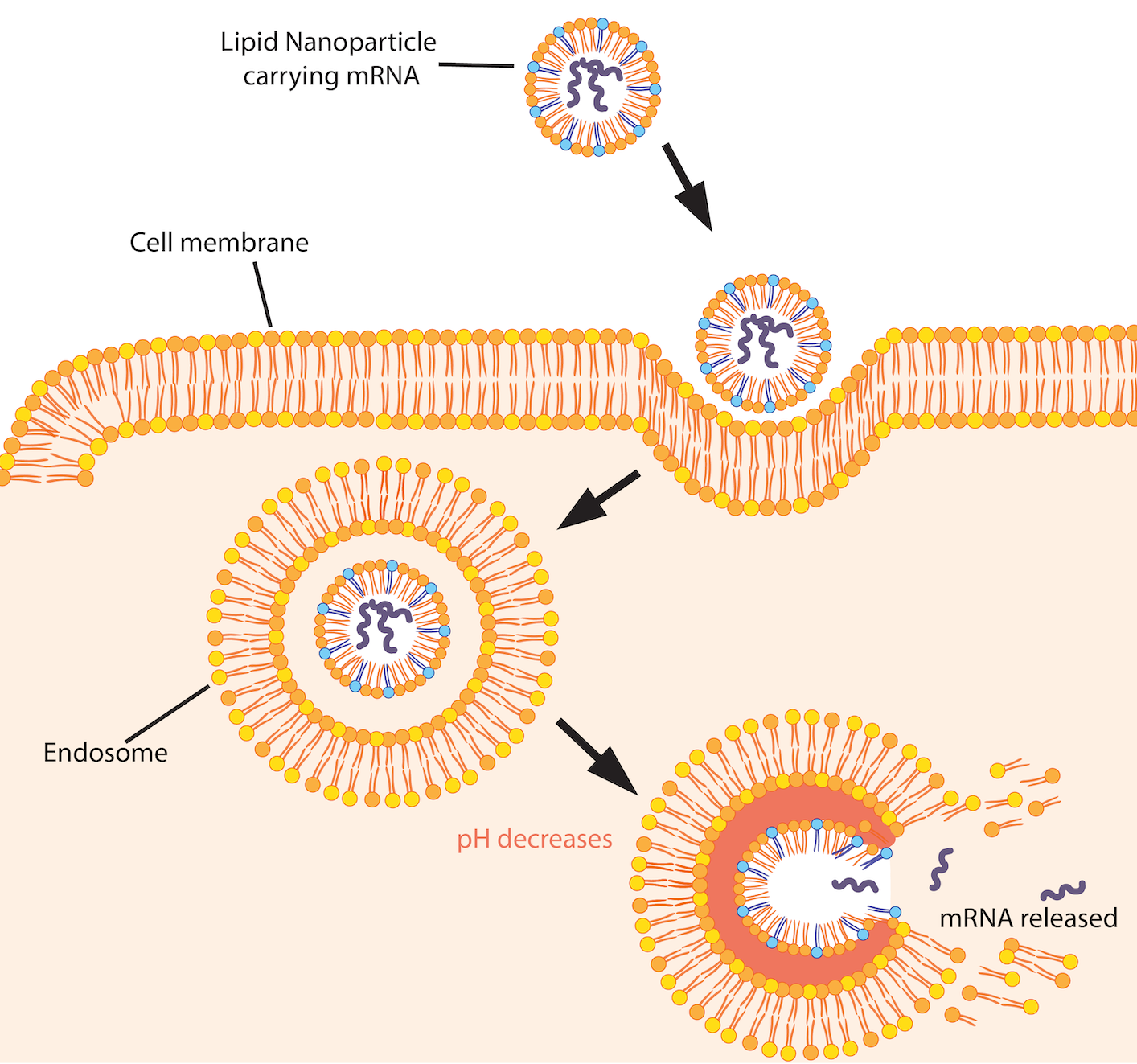
Lipid nanoparticles enter the cell by endocytosis – a process cells use to ingest particles. The cell membrane encapsulates the nanoparticle and pinches off, forming an “endosome” or pocket. As the endosome matures, its pH level drops to create an acidic environment. The lipid nanoparticle’s ionizable lipids become positively charged at low pH levels. They begin interacting with the negatively charged lipids in the endosomal membrane. This process disrupts the lipid nanoparticle, triggering mRNA release. Image by Nada Salem.
The lipid combinations comprising lipid nanoparticles can even be fine-tuned to selectively deliver them to different organs. Although all lipid nanoparticles contain ionizable lipids sensitive to the endosome’s acidic environment, scientists have observed that higher proportions of these ionizable lipids are optimal for targeting the liver. Permanently cationic (positively-charged) and anionic (negatively-charged) lipids better target the lungs and spleen, respectively.
Lipid nanoparticles have become an ideal candidate for delivering all types of RNA directly to cells, ushering in a new age of nanomedicine that scientists dreamed of decades ago. Canadian technology has paved the way for these nanoparticle delivery systems, from their inception to their current widespread use in life-saving medicine.
In the 1990s, University of British Columbia scientist Pieter Cullis pioneered research on pH-sensitive lipid nanoparticles. Companies co-founded by Cullis and built on his research led to the nanoparticles that made COVID-19 vaccines possible. Of those companies, Vancouver-based Acuitas is responsible for the lipid nanoparticles used in Pfizer-BioNTech’s vaccine. Precision Nanosystems (Vancouver), Entos Pharmaceuticals (Edmonton), and Providence Therapeutics (Calgary and Toronto) are also using lipid nanoparticle systems to develop COVID-19 vaccines. Some Canadian vaccines have already entered clinical trials.
In realizing its potential as a world leader in RNA medicine, Canada is laying the groundwork for more pharmaceutical independence. In February 2021, Precision Nanosystems was awarded $25 million by the Government of Canada to build an RNA Medicine Biomanufacturing Centre capable of producing 240 million doses of any mRNA vaccine per year. This centre is a vital step in facilitating Canada’s self-reliance in vaccine manufacturing and elevating our role in the future of RNA medicine – a future where we can finally reap the benefits of made-in-Canada medicine.
~30~
Banner image: CC0 image by torstensimon from Pixabay




Goood articles
very gooooooood
.Do you know? A fortune that you don’t use, is others wish