Esme Symons, Technology & Engineering editor
When we’re outside in the rain, we use raincoats and umbrellas to keep us dry. They are made of waterproof materials that have surface chemistries that are unfriendly to water. It takes less energy for the water molecules to be attracted to each than to the waterproof surface, so the rain beads up and rolls away.
Plants and animals can also benefit from staying dry and clean, and some of them take their water-repelling techniques a step further than our standard raincoats. An especially powerful ability to shed water is sometimes called “the lotus effect” because it is clearly observed on lotus leaves, which stay clean despite growing in muddy water. We see this effect in our backyards on butterfly wings, moth wings, and plant leaves including those of aspen trees and nasturtiums.
Scientists and engineers want to understand the water-repellant properties of these natural surfaces so they can build something that works the same way. These designs are an example of biomimicry: designing a technology inspired by nature. A well-known case of biomimicry is Velcro, whose hooks and loops stick together like burrs on dog fur. Other examples include gecko-foot-inspired adhesives, shark-skin-inspired antibacterial surfaces, and kingfisher-beak-shaped high-speed trains.
So, what’s behind “the lotus effect”?
The secret to superhydrophobicity
First, let’s describe hydrophobicity. The word “hydrophobic” comes from root words that mean “a fear or hatred of water”. In materials science, it refers to a material that repels water. We can measure how strongly a material repels water, or how hydrophobic it is, by placing a droplet of water on the surface and measuring the angle between the droplet and the surface.
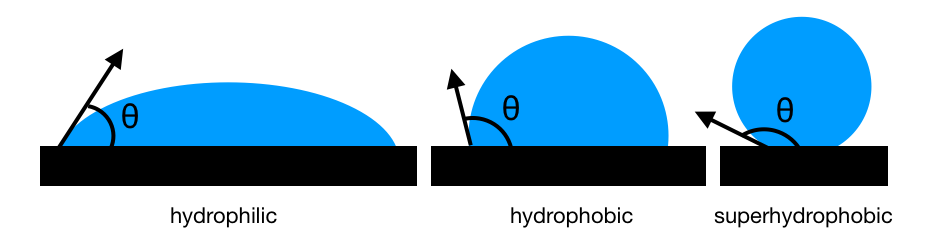
The contact angle of a water droplet sitting on a surface is an indication of whether the water and the surface repel or attract each other. Image credit: Esme Symons
The angle θ is measured at the point where the droplet, surface, and air all meet. It is measured from the horizontal surface through the droplet to the side of the droplet touching the air. The bigger the angle θ is, the more hydrophobic the surface – as if the water is uncomfortable sitting on the surface, so it’s touching it as little as possible. An angle less than 90 ° means the surface is hydrophilic, and easily wet by water. If the angle is greater than 90°, the material is hydrophobic, and repels water a moderate amount (like a raincoat does). If the angle is greater than 150 °, the material is very repellent to water, and is called superhydrophobic (or ultrahydrophobic).
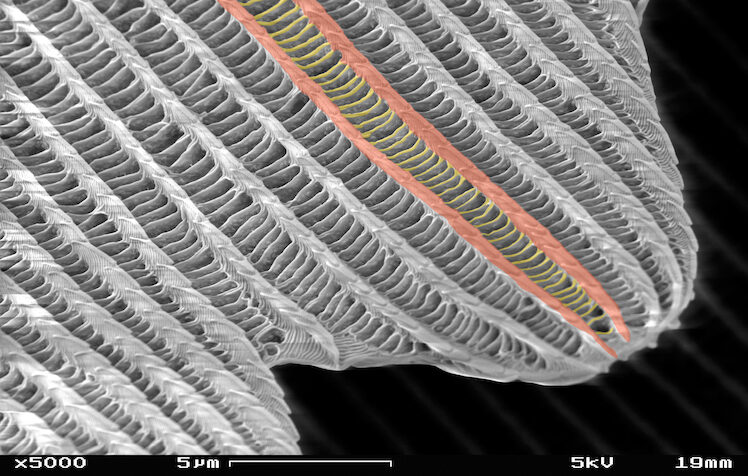
At 5000x magnification, we can see two sizes of texture on a butterfly wing.
Image credit: SecretDisk, CC BY-SA 3.0, modified
The trick to biological superhydrophobic materials lies in their surface texture. Butterfly wings are covered in scales made of a biological polymer called chitin. Chitin is inherently hydrophobic, but the texture on each scale bumps the water-repelling ability up even further. This texture can be seen at high magnification with a scanning electron microscope. The image above shows a single butterfly scale with micro-scale ridges (highlighted in red) and nano-scale bridges (highlighted in yellow) that connect the micro-scale ridges perpendicularly. The multi-scale texture of the scale, where some features are micrometre-sized and others are nanometre-sized, is what transforms hydrophobic chitin into superhydrophobic butterfly wings.
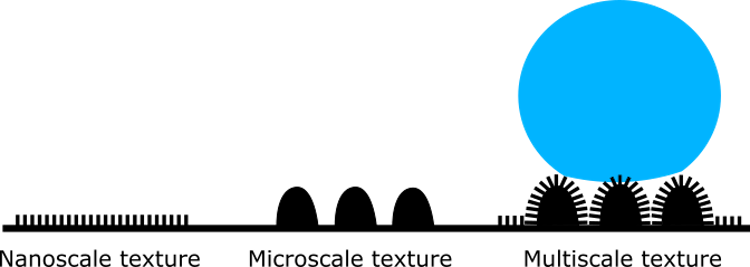
A superhydrophobic multi-scale texture consists of a nanometre-sized structure along with a micrometre-sized structure. Image credit: Munir.ashraf, CC0 1.0, modified
Combining these two different scales of texture affects the wetting state of the material, or how water interacts with it. Water can sit on a rough surface in two possible ways: the Wenzel state, where the water and surface are in complete contact, or the Cassie-Baxter state, where pockets of air reduce the contact area between the water and the surface. On a superhydrophobic surface, the combination of micro- and nano-scale textures puts the water into the Cassie-Baxter state, and the water can easily roll around on the surface.
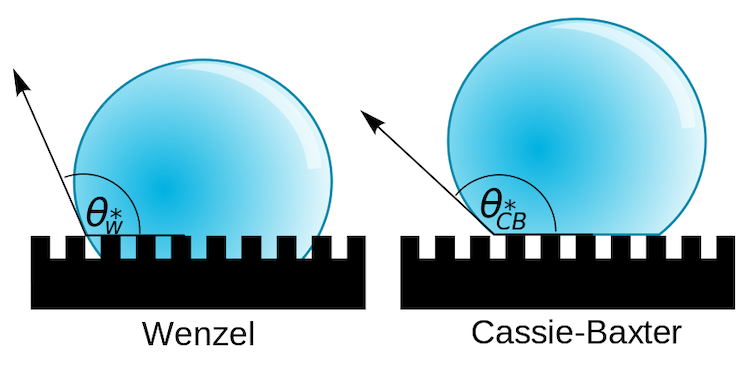
Two possible wetting states for a droplet on a textured surface. Image credit: Vladsinger, CC BY-SA 3.0, modified
How superhydrophobicity could be used in our everyday lives
Jason Tam, one of the researchers in a University of Toronto group working on superhydrophicity inspired by Canadian aspen tree leaves, says, “There are many exciting applications of superhydrophobic surfaces. For instance, […] the self-cleaning property. Dirt [or] dust accumulated on a superhydrophobic surface can be easily removed by a splash of water or rain droplets; the water will roll off the surface and carries the dirt away as well, leading to a clean and dry surface [sic]”. Tam notes that this could reduce the amount of time and labour needed to clean windows, especially on skyscrapers with many hard-to-reach stories.
Another possible application, particularly relevant to cold countries like Canada, is to limit ice formation on structures such as power transmission and communication towers or airplanes. When ice isn’t an issue, repelling water can still mean avoiding rust and corrosion, prolonging the life of metal structures.
Applying this technology to fabric would allow for superhydrophobic clothing, tents, and other soft items. Researchers in Montreal have developed a coating process for cotton fibres that gives the normally absorbent fabric superhydrophobic properties.
Durability issues
To imitate a butterfly wing or lotus leaf and create a superhydrophobic surface, we need to take a hydrophobic material and add multiscale texture to it. Methods for creating the texture include moulding, etching, and coating. Unfortunately, this critical surface texture can easily wear off, making durability a major issue. Tam explains one approach to this challenge: “To address this problem, we developed a superhydrophobic composite coating material comprised of nanocrystalline [nickel] matrix with embedded polytetrafluoroethylene [Teflon] particles. The former provides the strength and wear resistance while the latter provides the hydrophobic behaviour. […] Even when the composite material is subjected to abrasive wear during service, the hydrophobic particles will be continually exposed on the surface, providing long term non-wetting properties.”
Research into superhydrophobic materials continues and we could soon be seeing this super effective water-repellant property on anything that would benefit from staying dry. It’s all thanks to butterfly wings, lotus leaves, and the people who study them.
~30~
Banner image by haritama, CC-BY-3.0 https://commons.wikimedia.org/wiki/File:Mizutama_-_panoramio.jpg



