Joanie Tian, Chemistry editor
Given our ever-increasing demands for energy, it is more imperative than ever to find sustainable energy sources. Traditional methods of burning fossil fuels release greenhouse gases, specifically carbon dioxide, into the atmosphere. Greenhouse gases trap heat in the atmosphere, which leads to rising global temperatures resulting in rising sea levels and increasingly extreme weather conditions. Canada, the 38th-largest country by population, is the 7th-largest emitter of greenhouse gases. Thankfully, researchers have found another way to produce energy that is clean, sustainable, and reduces the amount of CO2 in the air.
Research published in the science journal Nature describes a process that could function like an “artificial leaf”. A team led by Dr. Yimin Wu at the University of Waterloo designed a method that turns carbon dioxide and water into methanol and oxygen by a procedure that is similar to photosynthesis.
Like photosynthesis, this reaction uses water, carbon dioxide, and sunlight. In photosynthesis, plants produce oxygen and glucose and use the energy stored in the glucose. In the “artificial leaf” process, however, methanol is produced instead of glucose, which can be used as a sustainably derived fuel.
This is not the first time that a method was devised to convert carbon dioxide and water into methanol. Wu’s method of methanol production is uniquely more efficient due to the use of cuprous oxide as a photocatalyst. He creates the cuprous oxide particles by heating up a mixture of copper acetate, sodium hydroxide, glucose, and sodium dodecyl sulphate to 60 °C for an hour.
A catalyst is a compound that makes it easier for a reaction to occur without getting used up in the reaction itself. The ‘photo’ part of the word photocatalyst means that it catalyzes a reaction by using light as its energy source. Cuprous oxide catalyzes the reaction by converting light surrounding water and carbon dioxide molecules into energy that the molecules can then use to help the reaction proceed. Wu’s method is currently the most efficient photocatalytic process for turning carbon dioxide into methanol.
To create the methanol, a stream of carbon dioxide gas is bubbled into water containing the photocatalytic cuprous oxide particles. This is done in the presence of sunlight. Wu’s team proposed the chemical reaction CO2 + 2H2O Ã CH3OH + O2 to describe this process.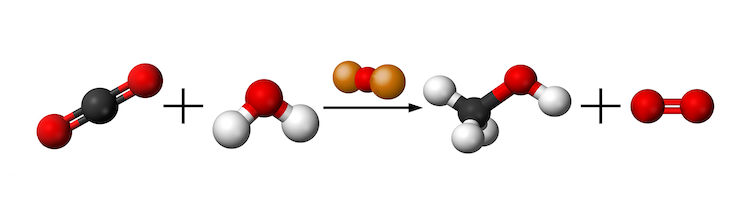
The advantages of Wu’s method are that it produces more consistent results at a higher efficiency than other methods while also using common materials. In addition, many of the processes used to manufacture methanol suffer from issues such as the need to use and store secondary energy sources such as electricity.
Methanol has many practical applications. It can be used to form other compounds such as formaldehyde, acetic acid, olefins, dimethyl terephthalate (DMT), and dimethyl ether (DME). These are commonly used in industry to create products like glues, preservatives, plastics, and cosmetics. There are also many benefits to using methanol as a fuel. For example, it is less flammable than gasoline, and methanol fires are easily put out with water. It is already being used as marine fuel because it produces emissions that are less harmful to the environment.
Some places in Canada are using carbon capture and storage methods (CCS) to reduce atmospheric carbon by storing it underground. Using this “artificial leaf” solution goes a step further, putting that captured carbon to use by converting carbon dioxide into methanol. There are other nature-mimicking projects of its kind, too. Researchers are looking into making microbes photosynthesize, making fuel out of carbon dioxide, and even other “artificial leaf” reactions. At the end of the day, it seems that the best way to protect nature is to learn from it.
~30~




