Lawrence Cheung, Chemistry co-editor
The widespread use of illegal drugs in British Columbia is chilling. It is a growing epidemic and the number of drug related deaths has increased each year since 2012. The situation is so bad that B.C.’s health minister, Terry Lake, has asked the federal government to declare a national public health emergency to help fight the drug overdose situation. From January to June of 2016, B.C.’s Coroners Service reported 238 illegal drug overdoses; that’s a 250% increase from the same period in 2015. Fentanyl has been connected to more than half of those deaths.
Fentanyl was designed to relieve pain but in the last couple of years it has received extensive media exposure as an illicit drug. A recent UN report found that Canada consumes the most prescription opiods per capita of any country in the world. People start with prescriptions from doctors but turn to criminal sources as they get hooked. The surging use of fentanyl as a narcotic can be attributed to its low cost and high potency. There is still a wide variety of street drugs, other than fentanyl, linked to BC’s overdose problem. How do police and forensic toxicologists differentiate one back alley drug from another?
Canada consumes the most prescription opiods per capita of any country in the world.
It is possible to identify fentanyl, or any other drug, using a two-step process. A high performance liquid chromatography (HPLC) separates a chemical mixture into its individual ingredients. Once the ingredients are separated, they are individually characterized by a mass spectrometer, which helps determine the amount of each component and its identity.
Let’s examine how an HPLC can separate the components of a fentanyl tablet. The tablet is dissolved in a solvent and then injected into the HPLC. A different solvent from the HPLC reservoir is continually pumped through the system and carries the injected mixture to a separating compartment called a column. The column contains various chemical components that are fixed in-place along its walls. These chemical components temporarily interact and bind with the ingredients in the test mixture. Each ingredient binds differently and the extent of binding depends on the chemical characteristics of the column component and the mixture ingredient.
It is possible to identify fentanyl, or any other drug, using a two-step process.
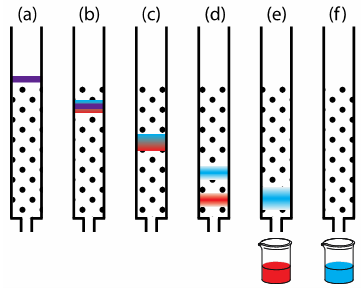
Think of the binding process as analogous to gluing different materials together. Carpenter’s glue can stick two pieces of wood together really well, but it doesn’t work as well when you try to glue wood and metal. In the HPLC, the injected ingredients don’t just bind with the column chemicals; they also bind and interact with the moving solvent. The interactions that each ingredient has with the column chemicals and moving solvent allow them to separate from each other, as seen in this figure.
Figure 1: The red and blue compounds can be separated because the red compound interacts less strongly with the stationary phase than the blue compound does.
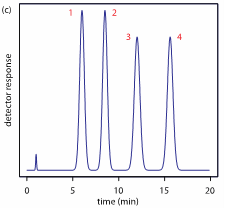
An ingredient’s retention time is the amount of time it spends in the HPLC from the time it is injected to the time it exits the column. Chemicals that have relatively weak interactions with the column exit quickly; chemicals with stronger interactions take longer to exit. The report produced from an HPLC analysis is called a chromatogram, and it displays each detected chemical as a function of its retention time in the HPLC.
Figure 2: An example chromatogram with the retention time displayed on the X-axis
Each peak in the chromatogram represents a chemical that has been separated from the test substance, and the area of each peak represents a quantitative measure of the amount of that chemical present in the test mixture. A chromatogram of a fentanyl tablet would show all of the components in the tablet. One would expect the fentanyl peak to be small compared to other ingredient peaks because the active ingredient in a drug is usually so potent that only minute quantities are needed. The remaining peaks represent the other ingredients that comprise the tablet.
By having a mobile mass spectrometer, ANKORS hopes to test recreational drugs at various events throughout the province
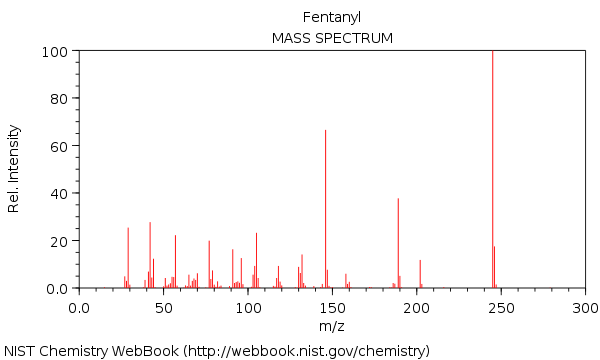
The second stage of the instrument is the mass spectrometer. Mass spectrometers produce ions from molecules by bombarding them with electrons. This involves a high velocity electron colliding with a molecule, which dislodges an electron from the molecule to produce a positively charged ion. This positive ion travels towards a mass analyzer; on its way the positive ion can fragment into smaller ions. The mass analyzer serves as a filter to separate ions; only ions of a certain mass travel pass the analyzer and onto the detector. A detector determines the mass of all the ions that make it past the analyzer – hence the name ‘mass spectrometer’. The report produced by the mass spectrometer shows the various signals of detected ions that result from the fragmentation process. Molecules fragment in a very characteristic way that allows their structure to be determined.
Figure 3: A mass spectrum of fentanyl
When the HPLC is linked to the mass spectrometer, it is possible to separate a mixture into its components and then analyze their mass and quantity. As a consequence, commercial products can be identified with a high degree of certainty because they are made from multiple ingredients that are used in specific quantities.
Law enforcement isn’t the only organization using this technology. Music festivals, like Shambhala, are a common place for recreational drug consumption. The Shambhala event organizers have teamed up with the AIDS Network Kootenay Outreach and Support Society (ANKORS), a non-profit organization that provides harm reduction services at numerous festivals, to raise awareness about the dangers of fentanyl. They currently have a crowd-funding campaign aimed at purchasing a portable mass spectrometer. By having a mobile mass spectrometer, ANKORS hopes to test recreational drugs at various events throughout the province and lower the number of fentanyl overdoses – just another way in which high-tech analyses are moving out of the lab and onto the streets.
Header image: Pixabay CC0 Public Domain