By Lauryn Doherty, guest contributor
Canadians are living longer. In 1950, our average life expectancy was about 68 years; in 2023, it has risen to 83 years. Unfortunately, the human body becomes more susceptible to disease as it ages. Alzheimer’s disease is a particular concern because approximately 76,000 new cases are diagnosed in Canada every year. Speaking at the Meeting of the Peripheral and Central Nervous System Drugs Advisory Committee, Lynn Kramer, the chief clinical officer of Eisai Inc stressed that “the complexity of care and cost burdens rise as the disease worsens, with severe impact on patients and their families.” This is a grim prognosis, but some researchers think that our understanding of this devastating disease, and our ability to treat it, is at hand.
What causes Alzheimer’s disease?
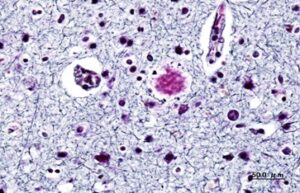
This histology staining of the amyloid beta plaques associated with Alzheimer’s disease clearly shows the plaques which appear as dark, dense nodules within the brain tissue. Image: Wikimedia Commons, CC SA 3.0.
This histology staining of the amyloid beta plaques associated with Alzheimer’s disease clearly shows the plaques which appear as dark, dense nodules within the brain tissue. Image by KGH. Creative commons.The amyloid hypothesis remains the leading explanation for what causes Alzheimer’s disease. This hypothesis is based on the presence of amyloid-beta proteins in the brains of autopsied patients with Alzheimer’s disease.
Why amyloid-beta proteins form in the first place is not fully understood, although research points to a combination of genetic and environmental factors. Images of affected brains show that as the disease progresses these proteins clump together, causing the formation of dense amyloid-beta plaques. These plaques appear to form in the spaces between neurons. Overtime this causes overcrowding in the brain and leads to the death of nerve cells and the loss of brain mass.
Collectively, these brain abnormalities are responsible for the feelings of confusion and mood alterations associated with Alzheimer’s patients.
Monoclonal antibody therapy
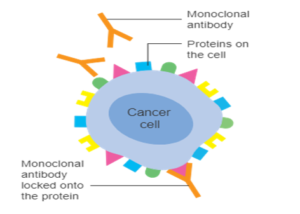
Monoclonal antibodies are designed to target specific antigens and mark them for destruction. In this example, the monoclonal antibodies are designed to target the HER2 receptor overexpressed on breast cancer cells. Image: Cancer Research UK.
Over the past 40 years, the emergence of monoclonal antibody therapy has revolutionised how we think about and treat complex diseases. The prevalence of monoclonal antibody therapy is clear when we look at its extensive use in clinical practice today. Notably, these diverse therapies are used to treat everything from severe cases of Covid-19 to HER2+ breast cancer.
Using our own body’s immune system as a blueprint, these highly specific therapies mimic how our immune cells naturally function when foreign invaders, such as bacteria, viruses or fungi, breach our body’s protective barriers and enter our system. Our immune system recognises unfamiliar antigens on the pathogen’s surface and responds by eliciting a counterattack— it produces antigen-specific antibodies that coat the invader and mark it for destruction. Using these clever principles, researchers have designed synthetic antibodies that can target a wide range of markers, including the HER2 receptor in the case of breast cancer or the SARS-CoV-2 spike protein in the case of Covid-19.
Now, researchers are considering how monoclonal antibodies could be used to target and destroy the amyloid-beta plaques that accumulate in the brains of patients with Alzheimer’s disease.
A new Alzheimer’s disease drug
Lecanemab is a monoclonal therapy designed to slow the development of Alzheimer’s disease by preferentially targeting amyloid-beta proteins in the brain to lower the level of plaques. Following promising results from a clinical trial, it was approved in July 2023 for use in early Alzheimer’s case by the US Food and Drug Administration (FDA). Health Canada accepted a New Drug Submission for lecanemab as a treatment for early Alzheimer’s disease in May 2023, but has yet to approve it.
One of the main obstacles to creating drugs for the brain is the blood-brain barrier, which is infamously challenging to cross. This problem has stumped researchers for decades and has hampered the development of effective drug delivery systems. Luckily, lecanemab’s small molecular size means that it can easily pass through the blood-brain barrier compared to bulkier drugs such as solanezumab that are too large to travel through the barrier’s restrictive membrane.
Limitations to the therapy
Although monoclonal antibody therapy holds great promise, there are limitations to this therapy. It does not cure Alzheimer’s disease, only slows its progression. This therapy is also costly. Lecanemab is listed at US$26,500 per year, representing a steep jump in price from traditional but less effective Alzheimer’s disease therapies.
The side effects of this drug are another drawback. More than one fifth of patients who took lecanemab as part of their treatment regimen experienced brain swelling and bleeding. Aducanumab, another monoclonal antibody therapy developed for Alzheimer’s disease saw 40% of its recipients experience these side effects.
Currently, lecanemab is only approved by the FDA for mild cases of cognitive impairment and in definite cases of amyloid pathology. This means that there remains a significant unmet need for those patients with advanced disease or those who do not have amyloid-beta plaques, about 14% of all patients with mild-moderate Alzheimer’s disease.
Although lecanemab may not be the miracle drug that we hoped for, it represents a massive step forward in our ability to intervene in early disease progression and apply the brakes to memory deterioration and cognitive decline. This breakthrough also signals that novel biotechnology approaches may be the key to unlocking a whole new world of patient treatment and care.
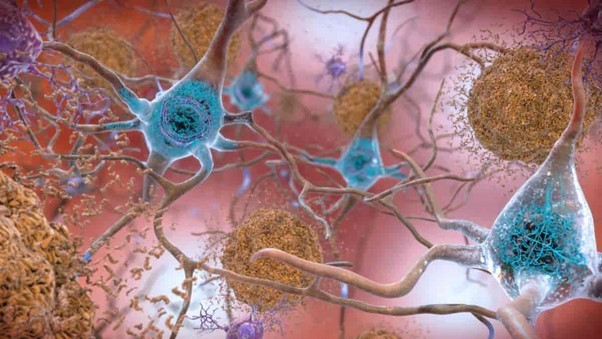
Feature image: Lecanemab is a monoclonal antibody therapy designed to target amyloid-beta plaques (shown in orange). These plaques surround neurons in the brain of an Alzheimer’s disease patient resulting in diminished cognitive capacity. Image: National Institute on Aging (NIH), CC0 1.0.