Komal Adeel, New Science Communicator
HIV is pretty young disease. Unlike malaria, which was with us when humans first migrated out of Africa some two million years ago, or tuberculosis, which has been found in the bodies of ancient Egyptian mummies, HIV infections did not exist in humans until the 20th century.
However, in this relatively short time period, HIV has become one of the biggest epidemics of our time. Over the course of the epidemic, HIV infected over 70 million people and killed 35 million. Though advances in treatment have meant less death, there is still no cure to the infection, and people living with HIV must stay on therapeutic drugs for life.
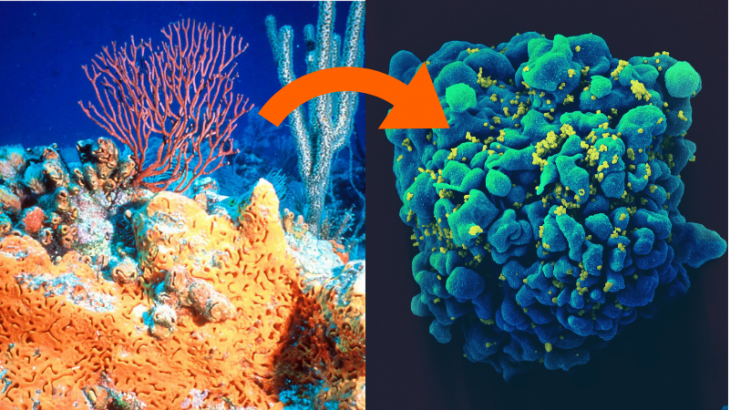
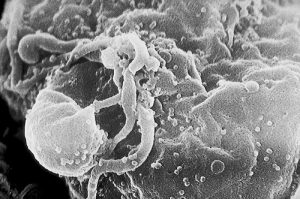
Left – An elephant ear sponge among other marine life (Wikimedia Commons); Right – An HIV-Infected T cell (NSAID via Wikimedia Commons)
We live in a time of medical breakthroughs and new technologies, and yet a cure to HIV remains elusive. However, at Simon Fraser University, Dr. Ian Tietjen and his research team are getting creative. They are looking for chemicals that overcome HIV, searching for possibilities in curious, seemingly unlikely places – including in marine organisms that thrive on the seafloor.
Why do these organisms hold promise? “Obviously a sponge doesn’t deal with HIV, but it may have had to deal with a pathogen that’s similar to HIV,” Dr. Tietjen explains. That potential could be harnessed to control HIV infection in humans.
What is it about HIV, however, that makes it so hard to cure? Why do Dr. Tietjen and his team have to look to the oceans’ depths to find a potential cure?
It has to do with a particularly sneaky virus that finds a way to hide in our own cells…
How our immune system battles the average virus
Viruses rely on the machinery of the cells they infect (host cells) to survive and reproduce. When a virus invades a cell, it starts making copies of itself. At this stage, little virus pieces, called antigens, float around in the cell, waiting to be put together.
However, the infected cells can create distress signals. To do this, they grab the virus particles, and hold them to the cell’s surface, where immune cells can perceive them and recognize them as foreign. One type of immune cell, the killer T cells, recognizes and destroys infected cells. Sacrificing host cells ensures that virus’s home, or reservoir,is destroyed and that the virus can no longer reproduce.
How HIV evades our immune system
So what makes HIV different? For one, it attacks immune cells – helper T cells to be exact. Helper T cells help other immune cells, including killer T cells, do their job. When HIV destroys them, it weakens our whole immune defense system.
HIV is also good at hiding. It is a retrovirus: it slices into a cell’s DNA and inserts its own genes. These genes – instructions to make new viruses – become integrated into the cell’s DNA. The virus can then ‘go to sleep’ – become latent – not making copies of itself.
Because the cell contains no floating viral antigens during latency, it cannot signal infection distress, and killer T cells will not recognize that the cell is infected. Even if killer T cells eliminate every single HIV-infected cell signalling distress, cells with latent HIV genes will escape the purge.
The virus-coding DNA instructions remain hidden, but can reactivate at any moment, even years later. This is why people living with HIV may experience no symptoms for long periods, then suddenly fall ill again. Because of HIV’s retroviral latency, HIV patients must stay on lifelong therapy. Antiretroviral drugs stop newly made viruses from infecting cells but, if patients stop taking these drugs – even after years of stable health – the virus genes in reservoir cells can activate and start producing the virus.
What is the solution?
If we could activate all the reservoir cells in a person infected with HIV so they start making viruses, the immune system would be able to recognize that these cells are infected and eliminate them.
This is what Dr. Tietjen’s team aims to do. Based on this “shock and kill” approach, they are seeking chemicals that can reactivate latent HIV inside reservoir cells – chemicals called latency reversal agents. The seafloor organisms that the team is probing are promising candidates for providing these agents. Because the organisms lack full immune systems, their modes of defense include antiviral chemicals. Many of these chemicals are active against HIV.
Humans have known of the therapeutic potential of marine organisms for centuries, but research into biologically active marine products began only in the 1950s, when Werner Bergmann identified compounds from marine sponges that lead to the development of anticancer drugs. Interest waned for many years as synthetic chemicals took the spotlight, but has since re-emerged, in large part because natural sources have high “hit” rates – they are more likely to yield promising leads – compared to libraries of synthetic compounds.
Dr. Tietjen’s lab works with libraries of compounds collected from marine invertebrates and microorganisms by other laboratories. The compounds’ sources range from bacteria from Papau New Guinea marine sediment to sea sponges from the Phillipines. Dr. Tietjen and his team test these compounds by putting them together with latent HIV-infected T cells to see whether the compounds reactivate HIV.
The lab has found that many of the effective latency reversal agents identified from these sources unwind DNA – including HIV DNA – making it more available to be read and turned into proteins. This means infected cells treated with the agent are more likely to make antigens, which can be displayed and recognized by immune cells.
However, these agents affect all DNA in all cells, not just HIV genes in reservoir cells. According to Dr. Tietjen, “They’re very nasty drugs…. HIV patients don’t like them, they’re hard to tolerate.” One of Dr. Tietjen’s goals is to find agents that function specifically. “We want to be able to target just the HIV genomic locus. We may then be able to use the agent at lower concentrations, and it wouldn’t be as toxic.”
Despite the prospects of latency reversal agents, Dr. Tietjen cautions that no HIV cure currently exists, and emphasizes that those living with HIV should maintain their antiretrovirals.
“We’re all working as hard as we can, and we will get there – I do believe that – but it’s a very difficult problem to solve,” he says. “And the cure’s not going to be, ‘Eureka! I found the cure!’ It’s not like you look in a test tube and that’s it. It’s all little steps here and there.” And, he says, with respect to all these many, little steps, we’ve come a long way.
You could say we’ve come all the way from the seafloor to the lab.

Members of the Tietjen lab and associated Brockman and Brumme lab at Simon Fraser University: (L-R) Mark Brockman, Silven Read, Tallie Kuang, Ian Tietjen, Tristan Markle, Natalie Kinloch, and Zabrina Brumme. Photo courtesy of Dr. Ian Tietjen
~30~
Simon Fraser University student Komal Adeel wrote this post as part of Science Borealis’s Spring 2019 Pitch & Polish, a mentorship program that pairs students with one of our experienced editors to produce a polished piece of science writing.
Read more about Pitch and Polish>